- 2021-04-16 发布 |
- 37.5 KB |
- 14页


申明敬告: 本站不保证该用户上传的文档完整性,不预览、不比对内容而直接下载产生的反悔问题本站不予受理。
文档介绍
2020医用口罩产品注册技术审查指导原则范文
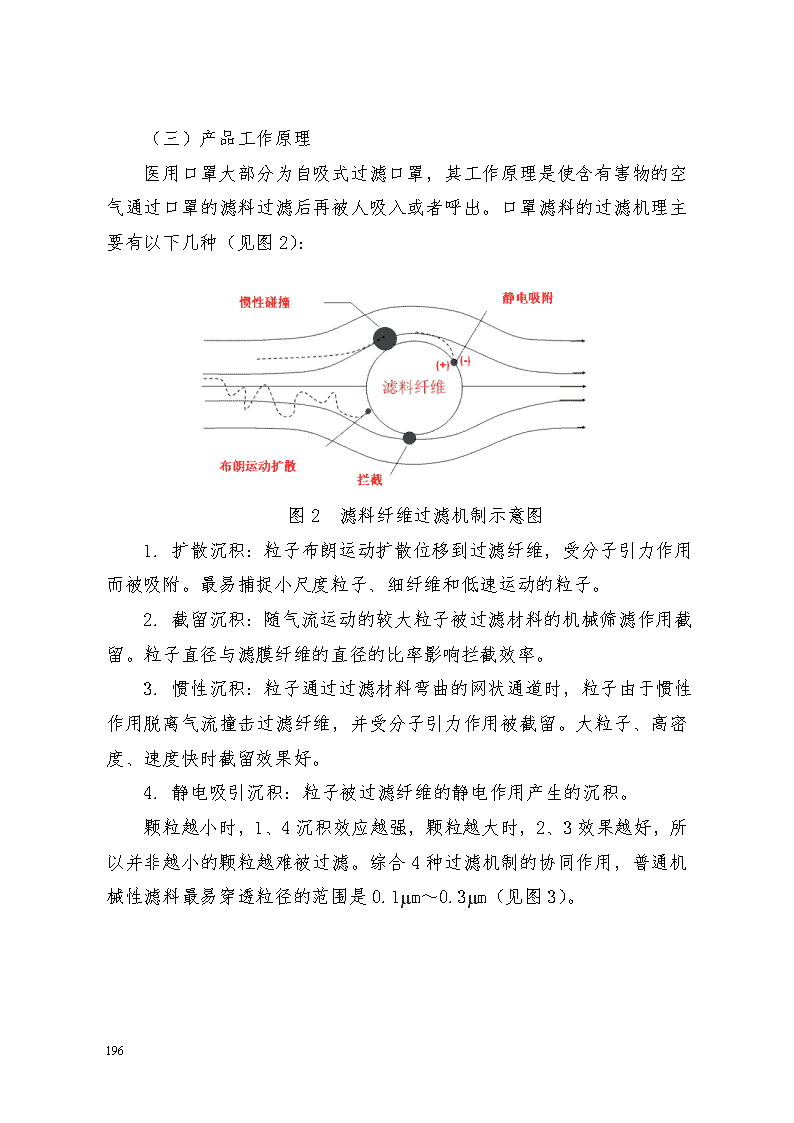
附件10 医用口罩产品注册技术审查指导原则 本指导原则旨在指导和规范医用口罩产品的技术审评工作,帮助审评人员理解和掌握该类产品原理/机理、结构、性能、预期用途等内容,把握技术审评工作基本要求和尺度,对产品安全性、有效性作出系统评价。 本指导原则所确定的核心内容是在目前的科技认识水平和现有产品技术基础上形成的。因此,审评人员应注意其适宜性,密切关注适用标准及相关技术的最新进展,考虑产品的更新和变化。 本指导原则不作为法规强制执行,不包括行政审批要求。但是,审评人员需密切关注相关法规的变化,以确认申报产品是否符合法规要求。 一、适用范围 本指导原则适用于医用防护口罩、医用外科口罩和医用普通口罩(一次性使用医用口罩)。《医疗器械分类目录》中管理类别为Ⅱ类,分类代号为6864。 本指导原则不适用于普通脱脂纱布口罩和各种含有杀菌、抑菌和抗病毒成分,预期用于抗菌抗病毒的口罩。 二、技术审查要点 (一)产品名称的要求 产品名称应以产品的预期用途和适用范围为依据,一般应为医用防护口罩、医用外科口罩和一次性使用医用口罩。 (二)产品的结构组成 1. 医用口罩一般由以下材料构成: 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 主体过滤材料:如聚丙烯熔喷布等。 其他材料:金属(用于鼻夹)、染色剂、弹性材料(用于口罩带)等。 2. 医用口罩一般有以下几种形式: 按照面罩形状可以分为平面形、鸭嘴形、拱形或折叠式等。 按照佩戴方式可以分为耳挂式、绑带式或头带式。 平面形 鸭嘴形(头带式) 拱形 折叠式 耳挂式 绑带式 图1 口罩形式 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — (三)产品工作原理 医用口罩大部分为自吸式过滤口罩,其工作原理是使含有害物的空气通过口罩的滤料过滤后再被人吸入或者呼出。口罩滤料的过滤机理主要有以下几种(见图2): 图2 滤料纤维过滤机制示意图 1. 扩散沉积:粒子布朗运动扩散位移到过滤纤维,受分子引力作用而被吸附。最易捕捉小尺度粒子、细纤维和低速运动的粒子。 2. 截留沉积:随气流运动的较大粒子被过滤材料的机械筛滤作用截留。粒子直径与滤膜纤维的直径的比率影响拦截效率。 3. 惯性沉积:粒子通过过滤材料弯曲的网状通道时,粒子由于惯性作用脱离气流撞击过滤纤维,并受分子引力作用被截留。大粒子、高密度、速度快时截留效果好。 4. 静电吸引沉积:粒子被过滤纤维的静电作用产生的沉积。 颗粒越小时,1、4沉积效应越强,颗粒越大时,2、3效果越好,所以并非越小的颗粒越难被过滤。综合4种过滤机制的协同作用,普通机械性滤料最易穿透粒径的范围是0.1µm~0.3µm(见图3)。 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 图3 滤料穿透率和粒径关系 (四)产品适用的相关标准 医用口罩产品应根据自身特点适用以下标准,但不限于引用以下标准: 表1 相关产品标准 标准编号 标准名称 GB/T 1.1-2009 标准化工作导则 第1部分:标准的结构和起草规则 GB/T 191-2008 包装贮运图示标志 GB/T 2828.10-2010 计数抽样检验程序 第10部分:GB/T 2828计数抽样检验系列标准导则 GB/T 14233.1-2008 医用输液、输血、注射器具检验方法 第1部分:化学分析方法 GB/T 14233.2-2005 医用输液、输血、注射器具检验方法 第2部分:生物学试验方法 GB 15979-2002 一次性使用卫生用品卫生标准 GB 15980-1995 一次性使用医疗用品卫生标准 GB/T 16886.1-2011 医疗器械生物学评价 第1部分:风险管理过程中的评价与试验 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — GB/T 16886.5-2003 医疗器械生物学评价 第5部分:体外细胞毒性试验 GB/T 16886.7-2001 医疗器械生物学评价 第7部分:环氧乙烷灭菌残留量 GB/T 16886.10-2005 医疗器械生物学评价 第10部分:刺激与迟发型超敏反应试验 GB 18279-2000 医疗器械 环氧乙烷灭菌确认和常规控制 GB 18280-2000 医疗保健产品灭菌确认和常规控制要求 辐射灭菌 GB 19083-2010 医用防护口罩技术要求 GB/T 19633-2005 最终灭菌医疗器械的包装 YY/T 0466.1-2009 医疗器械 用于医疗器械标签、标记和提供信息的符号 第1部分:通用要求 YY 0469-2011 医用外科口罩 YY/T 0615.1-2007 标示“无菌”医疗器械的要求 第1部分:最终灭菌医疗器械的要求 YY/T 0969-2013 一次性使用医用口罩 中华人民共和国药典二部(2010版) 产品适用及引用标准的审查可以分两步来进行。首先对引用标准的齐全性和适宜性进行审查,也就是在编写注册产品标准时是否引用了与产品相关的国家标准、行业标准,以及引用是否准确。可以通过对注册产品标准中“规范性引用文件”是否引用了相关标准,以及所引用的标准是否适宜来进行审查。此时,应注意标准编号、标准名称是否完整规范,年代号是否有效。 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 其次对引用标准的采纳情况进行审查。即所引用标准中的条款,是否在注册产品标准中进行了实质性的条款引用。这种引用通常采用两种方式,内容繁多的、复杂的可以直接引用标准及条文号,比较简单的也可以直接引述具体要求。 如有新版国家标准、行业标准发布实施,应执行最新版本的国家标准、行业标准。 (五)产品的预期用途 医用防护口罩适用于医务人员和相关工作人员对经空气传播的呼吸道传染病的防护。 医用外科口罩适用于医务人员或相关人员的基本防护,以及在有创操作过程中阻止体液和喷溅物传播的防护。 一次性使用医用口罩适用于佩戴者在不存在体液和喷溅风险的普通医疗环境下的卫生护理。 (六)产品的主要风险 医用口罩产品在进行风险分析时应符合YY/T0316-2008《医疗器械 风险管理对医疗器械的应用》的要求。 企业在进行风险分析时,至少应考虑表2中的主要危害,企业还应根据自身产品特点确定其他危害。针对产品的各项风险,企业应采取应对措施,确保风险降到可接受的程度。 表2 产品主要危害 危害类型 可能产生的危害 形成因素 控制措施 生物学危害 生物污染 产品没有消毒/灭菌或消毒/灭菌没有达到标准 严格控制消毒/灭菌工艺有明确的消毒/灭菌程序,每批进行消毒/灭菌效果检验 生物相容性 生产引入的外来有害物质没有被有效去除;环氧乙烷残留量超标 原材料入厂检验; 严格控制灭菌工艺 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 与产品使用相关的危害 不适当的标签 产品最小包装标记不清晰、不全面、不正确 标记印刷清晰正确; 标记内容按相关要求标记全面 说明书上的注意事项不全 如缺少详细的使用方法、缺少必要的警告说明 规范说明书 对一次性使用产品的很可能再次使用的危害性警告不适当 说明书中未包含只限一次性使用 规范说明书 功能失效引起的危害 不适当的预期用途表述 说明书中未能清楚表明产品用途 规范说明书 不适当的产品包装 生产、运输、搬运和储存过程中导致包装破损; 包装封口不严密; 包装材料选择不适当 严格控制包装工艺; 失去产品的完整性 产品各构件之间缝制、粘合不严密或材料本身存在破损达不到隔离要求 严格控制生产工艺、产品检验 产品对环境的危害 环境污染 生产环境污染产品,如外来的纤维、粉尘、细菌等其他杂质; 产品原材料受到污染; 储运环节污染产品 严格控制生产环境; 严格控制原材料采购、检验; 严格控制产品储运环节 (七)产品的主要技术指标 本条款给出医用口罩产品需要满足的性能要求 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — ,其他性能要求企业可参考相应的国家标准、行业标准,根据企业自身产品的技术特点制定相应的要求,但不得低于相关强制性国家标准、行业标准的有关要求。如有不适用条款(包括国家标准、行业标准要求),企业在标准的编制说明中必须说明理由。 如企业直接采用国家标准、行业标准作为产品标准的,应提交所采纳的国家标准或行业标准的有效文本及采标说明。采标说明应至少包括产品规格型号的划分、产品的结构组成、产品的管理类别、产品的出厂检测项目、完全执行此标准的承诺及其他应说明的内容。 如企业制定注册产品标准,则标准中应明确规格型号的划分、产品的结构组成等内容,且性能指标应能满足以下要求: 1. 医用防护口罩:应符合GB 19083-2010《医用防护口罩技术要求》; 2. 医用外科口罩:应符合YY 0469-2011《医用外科口罩》; 3. 一次性使用医用口罩:应符合YY/T 0969-2013《一次性使用医用口罩》要求。 (八)产品的检测要求 医用口罩产品的检测包括出厂检验和型式检验。 出厂检验项目至少应有以下项目:外观、结构与尺寸、鼻夹、口罩带、微生物指标、环氧乙烷残留量(若采用环氧乙烷灭菌)的要求。 型式检验应为产品标准的全性能检验。 (九)产品的临床要求 根据《关于印发豁免提交临床试验资料的第二类医疗器械目录(试行)的通知》(国食药监械〔2011〕475号),医疗器械生产企业在申报《豁免提交临床试验资料的第二类医疗器械目录(试行)》范围内产品注册时,可以书面申请免于提交临床试验资料,但应同时提交申报产品与已上市同类产品的对比说明。对比说明应当包括工作原理、产品材质、结构组成、主要技术性能指标、消毒/灭菌方法 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 、预期用途、是否家庭使用等内容。 (十)产品的不良事件历史记录 暂未见相关报道。 (十一)产品说明书、标签、包装标识 产品说明书、标签、包装标识应当符合《医疗器械说明书、标签和包装标识管理规定》(国家食品药品监督管理局令第10号)及相关标准的要求。 1. 医用口罩产品说明书或包装标识应至少包括以下内容: (1)产品名称、型号、规格; (2)生产企业名称、注册地址、生产地址、联系方法; (3)《医疗器械生产企业许可证》编号、《医疗器械注册证》编号、执行标准代号; (4)产品生产批号; (5)产品使用的原材料及结构、组成; (6)产品的主要性能; (7)产品的规格尺寸; (8)产品的适用范围; (9)注明“使用前应参见使用说明”; (10)注明佩戴方法,若口罩防护功能受正反面佩戴影响还应明确标识口罩正反面识别方法。应提示避免手部接触口罩内侧; (11)一次性使用产品应注明“一次性使用”字样或符号,禁止重复使用; (12)已消毒/灭菌产品应当注明消毒/灭菌方式及失效期; (13)应提醒使用者勿使用包装已损毁的产品; (14)产品贮存条件和方法; 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — (15)产品开封后应尽快使用的提示; (16)产品使用后需要处理的,应当注明相应的处理方法。 2. 医用防护口罩除应达到以上要求还应包含: (1)应注明使用前需进行的检查; (2)应提示佩戴适合性; (3)应给出口罩使用时间的建议; (4)应注明滤料级别或相关说明。 (十二)注册单元划分的原则 医用口罩产品的注册单元原则上以技术结构、性能指标和预期用途为划分依据。通常按照口罩的分类划分为医用防护口罩、医用外科口罩和一次性使用医用口罩三个注册单元。 (十三)同一注册单元中典型产品的确定原则 同一注册单元内,典型产品作为被检测的产品。典型产品是指能够涵盖本注册单元内全部产品工艺的一个或多个产品。 按照“同一注册单元内,所检测的产品应当是能够代表本注册单元内其他产品安全性和有效性的典型产品”的原则,抽取样品应能涵盖该注册单元全部产品的技术要求。面罩形状不同的口罩应分别进行密合性检测,如鸭嘴形口罩密合性检测不可覆盖平面形口罩。 三、审查关注点 (一)产品标准要求 应关注医用口罩产品注册标准编写的规范性,引用标准的适用性、准确性。如直接采用国家标准、行业标准作为产品标准的,应注意采标说明内容的完整性。 (二)产品技术报告要求 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 应关注医用口罩的质量控制要求,主要包括过滤材料和生产工艺。应对产品的过滤材料进行控制,明确过滤材料的来源及质量要求,材料应具有相对稳定的生产工艺及供货来源以保证产品的质量。 (三)产品说明书要求 应关注说明书中声称的产品结构、尺寸和其他技术信息应与标准及注册检测报告一致。应关注产品适用范围应与所采用的产品标准相符。应根据口罩类别保证说明书注意事项的完整性。 (四)注册检测的典型产品 应关注注册检测产品是否能够代表本注册单元内其他产品安全性和有效性。应注意医用防护口罩不同形式产品的密合性差异,例如立体口罩的密合性一般优于平面口罩。 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 医用口罩产品注册技术审查指导原则编制说明 一、指导原则编写的原则 (一)本指导原则编写的目的是用于指导和规范第二类医用口罩产品注册申报过程中审查人员对注册材料的技术审评。 (二)本指导原则旨在让初次接触该类产品的注册审查人员对产品原理、结构、主要性能、预期用途等各个方面有个基本了解,同时让技术审查人员在产品注册技术审评时把握基本的尺度,对产品安全性、有效性作出系统评价。 二、指导原则编写的依据 (一)《医疗器械监督管理条例》 (二)《医疗器械注册管理办法》(国家食品药品监督管理局令第16号) (三)《医疗器械说明书、标签和包装标识管理规定》(国家食品药品监督管理局令第10号) (四)《医疗器械标准管理办法》(国家药品监督管理局令第31号) (五)关于印发《境内第一类医疗器械注册审批操作规范(试行)》和《境内第二类医疗器械注册审批操作规范(试行)》的通知(国食药监械〔2005〕73号) (六)关于加强医用口罩监管工作的通知(食药监办械〔2009〕95号) (七)关于进一步规范医用口罩注册工作的通知(国食药监械〔2009〕755号) (八)关于印发豁免提交临床试验资料的第二类医疗器械目录(试行)的通知(国食药监械〔2011〕475号) (九)国家食品药品监督管理部门发布的其他规范性文件 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 三、指导原则中部分具体内容的编写考虑 (一)根据《关于进一步规范医用口罩注册工作的通知》(国食药监械〔2009〕755号)规定了口罩的分类并参考专家意见确定了各类口罩的适用范围。 (二)目前医用防护口罩、医用外科口罩和一次性使用医用口罩的相关国家标准或行业标准都已发布,所以产品的主要技术指标完全执行相关国家标准或行业标准。 (三)医用口罩的安全性、有效性只是其起到防护作用的一部分因素。口罩正确的使用及佩戴方法也直接影响了防护的效果。因此本指导原则在产品说明书的编写上给予指导,目的在于使说明书内容能够更加全面,明示出使用者需要的全部信息以避免口罩的误用,降低交叉感染的风险。目前,医护人员对于不同种类医用口罩的适用范围还没有十分明确的认识。应在说明书中清楚地注明口罩的适用范围并加强医护人员的培训工作。 (四)产品的主要风险参照YY/T0316-2008中附录E进行。 (五)产品的不良事件历史记录主要从国家药品不良反应监测中心数据库中查找。 (六)鉴于目前对医用口罩的过滤材料质量难以通过便捷有效的检验方式来控制,生产质量管理体系的规范运行是企业保证口罩产品质量稳定的主要手段,故在审查关注点中要求审核人员应关注过滤材料的生产工艺及供货来源。 四、相关参考资料 本指导原则在编写过程中也参考了美国食品药品管理局(FDA)的相关指导原则,例如2004年发布的《Guidance for Industry and FDA Staff:Surgical Masks – Premarket Notification [510(k)] Submissions》 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 — 和2007年发布的《Guidance for Industry and FDA Staff - Class II Special Controls Guidance Document: Filtering Facepiece Respirator for Use by the General Public in Public Health Medical Emergencies》。 五、指导原则编写人员 本指导原则的编写成员由北京市食品药品监督管理局医疗器械产品注册技术审评人员、行政审批人员、北京市医疗器械检验所专家、临床医学专家、预防医学专家、专业厂家代表共同组成,特别是北京市医疗器械评审专家委员会委员全程参与了本原则的研讨和制订,以充分利用各方面的信息和资源,综合考虑指导原则中各个方面的内容,尽量保证指导原则正确、全面、实用。 36年来,支月英坚守岗位,把爱意播撒在这青山绿水,让这份爱生根发芽,承载起贫瘠山村的绿色希望。Ellipticity of elbow pipes with diameters less than or equal to 150 mm must be not greater than 8% diameter of 200 mm or less shall not be greater than 6%. Wall thickness of the pipe wall thinning rate must not exceed the original 15%. Crease roughness: diameter 125 mm or less, shall not exceed 3 mm diameter less than or equal to 200 mm, not more than 4 mm. 7.3.9 simmering bending production of square steel tube extension, to use the whole tube bending. Interface if necessary, the welding position should be located in the middle of the vertical arm. 7.3.10 install expansion joints should be done. If design is not required, pipe compensator lengthen should conform to the requirements in the following table: square scale length δ x is equal to 1/2. Pre stretch tolerance: casing + 5 mm, +10 mm. 7.3.11 pipe form, location, spacing shall meet the design and specifications. 7.3.12 piping on back or return pipe at higher levels of the upper to automatic valve to install a drain valve below the horizontal parts. 7.3.13 supports and hangers of checks and the number 1) hanger installed must be checked before installation of supports and hangers part models, specifications of springs setting values, whether the materials meet the requirements of drawings and documents; 2) supports and hangers of material, size and accuracy shall conform to the provisions of the design drawings, material technical requirements should be consistent with national standards, industry standards and technical requirements related to the alloy spectrum review should be carried out; 3) pipe supports and hangers part plant product quality certificate for each variable spring supports, constant support hangers, dampers, damper function, there should be factory calibrated test— 207 —查看更多